Improve carbon capture efficiency with advanced instrumentation
The global urgency to reduce greenhouse gas (GHG) emissions and combat climate change is driving carbon capture, utilization and storage (CCUS) technologies to the foreground of industrial organizations’ corporate visions. As industries and governments strive to meet increasingly stringent environmental regulations and decarbonization goals, the need for efficient and cost-effective carbon capture methods is becoming increasingly paramount.
Among the available techniques, amine gas treatment has emerged as a leading contender, providing a promising solution for capturing carbon dioxide from industrial flue gases. However, effectively monitoring the efficiency and intricacies of this process poses a challenge.
This article breaks down the amine gas treatment approach, expresses the challenges in monitoring these systems, and describes the ways advanced instrumentation — particularly Raman spectroscopy — enhances the process, optimizing carbon capture efficiency to meet sustainability objectives.
Sweetening the stream
Amine gas treatment is a well-established chemical process that utilizes aqueous solutions of various alkylamines — known as amines, which function as solvents — to dissolve and remove carbon dioxide, hydrogen sulfide and other acid gases from gas streams. This process is also known as “sweetening” because it improves the odor of the processed products by removing “sour-smelling” hydrogen sulfide and other toxins. Amine gas treatment is widely employed in refineries, petrochemical plants and natural gas processing facilities because of its high carbon dioxide capture efficiency and proven methodology, dating back to 1930.
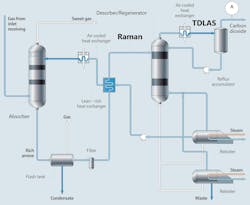
The amine gas treatment process can be broadly divided into three main stages: absorption, desorption and regeneration. During absorption, the flue gas containing carbon dioxide is introduced into an absorber column where it contacts the amine solution. The amines react with carbon dioxide, forming a chemical bond and effectively removing it from the flue gas stream (Figure 1).
The carbon dioxide-rich amine solution then moves to the desorber/regeneration column where it undergoes heating, causing the chemical bond between the amine and carbon dioxide to break. This releases the captured carbon dioxide, which can be further processed for storage or utilization, while the regenerated amine solution is recycled back to the absorber for reuse. Precise control of the temperature during this phase is crucial: low temperature can cause inadequate separation of carbon dioxide from the rich amine solution, while high temperature can produce premature degeneration of the amine.
Several factors contribute to the cost and efficiency of amine gas treatment. Key efficiency measures include the absorption rate of carbon dioxide, the energy required for regeneration and the overall capture rate. Optimization efforts often focus on minimizing solvent losses, reducing energy consumption and maximizing the carbon capture rate.
Along with providing obvious emissions reduction benefits, amine gas treatment also introduces business challenges. For example, the process can be energy-intensive, particularly during the regeneration stage, which increases operational costs. Additionally, amine solutions can degrade over time due to factors such as oxidation and thermal degradation, impacting long-term performance and necessitating solvent replenishment.
Monitoring challenges
Efficient and real-time monitoring of amine systems is crucial for maintaining optimal performance and ensuring solvent health. However, traditional monitoring methods require offline sampling and laboratory analysis, which are time-consuming and labor-intensive. In addition, they pose safety risks to personnel handling potentially hazardous amine solutions. The lack of real-time data hinders process control and optimization, leading to inefficiencies and increased operational costs.
Additionally, the delays between sample collection, analysis and corrective actions creates a sluggish treatment/feedback loop, which can have detrimental consequences. For instance, oversight of critical fluctuations in amine concentration or degradation products can cause suboptimal acid gas removal efficiency.
Moreover, the limitations of offline measurements hinder efforts to optimize energy efficiency for the process. Because real-time data on amine properties — such as solvent loading and regeneration heat duty — is unavailable, operators struggle to fine-tune parameters for maximum capture efficiency while minimizing energy consumption. This not only impacts economic viability, but it also hinders progress towards achieving decarbonization goals.
The limitations of traditional methods underscore the need for advanced, inline analytical techniques that can provide continuous and accurate measurements of key parameters within amine systems. These parameters include amine concentration, carbon dioxide loading, solvent quality and the presence of degradation products. Real-time monitoring enables operators to make informed decisions regarding process adjustments, solvent replenishment and maintenance schedules, improving the overall efficiency and cost-effectiveness of carbon capture operations.
Raman spectroscopy enhances monitoring
Raman spectroscopy is a powerful tool for addressing these and other needs, providing non-invasive, inline and real-time analysis of chemical composition for various industrial processes, including amine gas treatment. This technique exploits the inelastic scattering of light by molecules to provide detailed information about vibrational and rotational modes, which are unique to each chemical species and can be used to identify gas compositions.
When a beam of light interacts with a substance, a portion of the light is scattered with slightly altered energy. This shift, meticulously captured by a Raman spectrometer, creates a unique fingerprint for each molecule present, which can be used not only to identify the sample’s composition, but also to quantify each components’ precise concentration. Performing these measurements with inline Raman instruments for continuous monitoring maximizes process efficiency by providing (Figure 2):
- High sensitivity and specificity: Unlike traditional measurement methods that struggle to identify complex mixtures, Raman spectroscopy exhibits exceptionally high sensitivity and specificity. It can readily detect and quantify even trace amounts of different components in these mixtures, ensuring accurate measurements of amine concentration, carbon dioxide loading and degradation byproducts. These granular details help ensure accurate measurements, crucial for optimizing overall amine system performance.
- Portability and ease of integration: Inline Raman analyzers can be readily integrated into existing process lines without the need for extensive modifications, facilitating real-time monitoring and control (Figure 2). This allows for responsive, continuous monitoring without compromising the integrity or efficiency of the gas treatment process, and it provides process insights for informed decisions and optimal control.
- Predictive capabilities: By tracking changes in the Raman spectra over time, analytics software can identify varying process conditions and provide valuable insights into the behavior of amine systems, enabling predictive control and optimization of the carbon capture process. This empowers operators to anticipate potential issues and proactively adjust parameters for optimal carbon capture efficiency, with the ability to foresee amine solvent degradation before performance is significantly impacted.
A study conducted at the University of Edinburgh1 confirmed the reliability and accuracy of inline Raman spectroscopy for monitoring amine systems by comparing Raman measurements with traditional titration methods. This demonstrated that Raman spectroscopy can accurately track changes in carbon dioxide and amine concentrations even under varying process conditions. These findings highlight the potential of Raman spectroscopy for real-time monitoring and control of carbon capture processes, paving the way for improved efficiency and reduced operational costs.
Combining TDLAS with Raman spectroscopy to orchestrate optimal carbon capture
Tunable diode laser absorption spectroscopy (TDLAS) is another valuable measurement technology that complements Raman spectroscopy to optimize carbon capture by continuously monitoring carbon dioxide concentration in the flue gas stream after amine treatment.
TDLAS exhibits a fast response time, tracking carbon dioxide concentration fluctuations in near-real time. As a result, sudden spikes and dips are immediately detectable, so operational teams can swiftly investigate and rectify developing issues before they cause problematic consequences. Additionally, accurate and precise TDLAS data is vital for assessing the overall effectiveness of the carbon capture process.
Integrating both Raman spectroscopy and TDLAS into control systems provides operators with a comprehensive understanding of the amine system and the carbon capture efficiency, enabling real-time optimization, enhancing performance and minimizing energy consumption and solvent losses. This information empowers operators to track both amine solvent health via Raman measurement and carbon dioxide purity via TDLAS, identify areas for improvement and fine-tune parameters to maximize process efficiency.
A path to efficient carbon capture
Amine gas treatment is one of the most efficient and cost-effective methods of carbon capture, especially when coupled with advanced inline monitoring techniques — such as Raman spectroscopy and TDLAS — paving the path toward sustainable operations. This pairing provides the means to maximize carbon capture while minimizing wasted energy and solvent losses.
Real-time monitoring empowers processors to optimize their gas treatment processes, contributing to a more sustainable and environmentally-friendly approach to carbon capture. And as industries strive to meet decarbonization goals and comply with environmental regulations, embracing these technologies will be crucial in mitigating GHG emissions and transitioning toward a cleaner future.
Alan Garza is the product marketing manager for the advanced analysis product lines at Endress+Hauser. He began his career at Endress+Hauser as a rotational engineer where he developed multiple instrumentation technologies. Alan was also part of the inside sales team where he championed gas analytics and developed as an applications engineer. His background also includes business development and operations management. Alan holds a BS in Mechanical Engineering Technology from the University of Houston.
[1] Akram, M, Jinadasa, MHWN, Tait, P, Lucquiaud, M, Milkowski, K, Szuhanszki, J, Jens, K, Halstensen, M & Pourkashanian, M 2020, 'Application of Raman Spectroscopy to Real-Time Monitoring of CO2 Capture at PACT pilot Plant; Part 1: Plant operational data', International Journal of Greenhouse Gas Control. https://doi.org/10.1016/j.ijggc.2020.102969, https://doi.org/10.1016/j.ijggc.2020.102969
About the Author
Alan Garza
Product marketing manager for the advanced analysis product lines at Endress+Hauser
Alan Garza is the product marketing manager for the advanced analysis product lines at Endress+Hauser. He began his career at Endress+Hauser as a rotational engineer where he developed multiple instrumentation technologies. Alan was also part of the inside sales team where he championed gas analytics and developed as an applications engineer. His background also includes business development and operations management. Alan holds a BS in Mechanical Engineering Technology from the University of Houston.