How to ensure reliable pressure transmitter measurement in pharmaceutical applications
In pharmaceutical and biopharmaceutical production facilities, pressure measurement is critical to sterile and precise batch processes of material such as vaccines, commodity chemicals or specialized cellular tissue. Pressure transmitters perform the essential function of tracking the pressure within these closed process systems. Any errors or deviations from the set standard might mean a batch must be discarded because it more than likely will not meet stringent government regulations.
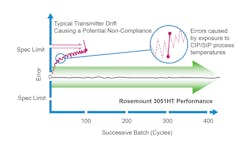
Where transmitters can become compromised is during the mandatory cleaning cycles between batches. The extreme heat of steam-in-place cycles exposes the transmitter to high pressure and temperatures that can lead to errors in the transmitter data. If transmitter drift is significant enough, which it is likely to be, it will cause batch inconsistencies.
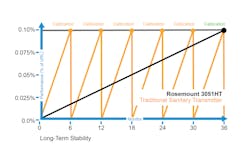
Legacy pressure transmitters tend to fail, needing recalibration to ensure performance is back to within specifications. If that does not work, they will need to be replaced, which is a significant expense, especially if it becomes a recurring expense. A new device was recently introduced to the market, an advanced hygienic pressure measurement device, which can withstand many clean-in-place (CIP) and sterilization-in-place (SIP) cycles. The device — the Rosemount 3051HT Hygienic Pressure Transmitter — dramatically reduces transmitter drift and provides long-term stability to pressure measurement performance.
Biopharma batch processes rely on a key indicator
Pressure measurement is a key indicator in the types of batch processes found in drug manufacturing and bioprocess applications. The bioreactors used are generally made from stainless steel to support the required biologically active environment. The bioreactor provides a homogenous environment for the cells used, ensuring that pH and oxygen levels remain constant. Establishing and maintaining a prescribed set of conditions to ensure the same quality batch after batch is the goal of this type of closed system. Ensuring a sterile environment along with other set parameters means the entire process must be monitored throughout each step.
Cells and a sterile nutrient medium provided at the start of cultivation are the ingredients for batch bioprocess. When producing enzymes, the enzymes and their substrates are used at the beginning of the process, and no additional nutrients are added, which means contamination is extremely unlikely. After harvesting the end product, the reactor must be cleaned and sterilized before starting the next batch. The advantages of utilizing batch processes are many, but the success of batching depends on a highly sterile and controlled environment. Each batch is its own individual production.
To ensure the success of batch processes, hygienic pressure transmitters are used in a range of reactor types, such as fed-batch or continuous-perfusion bioreactors, to continuously monitor the batch pressure. Proper batch pressure must be maintained to avoid potentially dangerous conditions from arising, such as tanks overflowing or becoming over-pressured, which causes safety concerns. When chemical reactions do not occur in optimal conditions, it can cause the bioprocess to underperform or fail altogether, which can lead to lower product quality or a total scrapping of a batch as useless. Similarly, if off-gas filtration is not adequate, it can lead to wasted product or require additional processing to bring the batch up to standard. Avoiding these types of problems requires best-in-class equipment.
Avoiding heat damage to equipment in cleaning cycles
After a batch is complete, running cleaning and sterilization cycles requires running fluids at high temperatures through the device. However, cleaning fluid can reach temperatures of up to 300˚F, which can negatively affect sensing devices throughout the reactor. When a pressure transmitter is subjected to these kinds of extreme temperatures, it can become offset, introducing errors into the measurement data (see Figure 2). Running CIP and SIP cycles are required to fully clear any biological materials from the previous batch from the system. In essence, returning the bioreactor back to a sterilized state before starting a new batch.
Cleaning cycles take time, and the pressure transmitter needs time to recover after cleaning to get back to its normal performance range. After a steam-in-place cleaning cycle, the transmitter might not initially return to working pressure specifications. The return might be gradual, or it might not happen at all without performing costly maintenance to recalibrate and return the device to the originally calibrated settings.
With legacy equipment, it is especially harder to know how soon the measurement data can be accurate again after CIP and SIP cycles. How many times can CIP and SIP cycles be run before a transmitter is no longer meeting specifications? Are there guidelines in the industry that include standards for repeat recalibration or replacement after a certain number of cycles? This information becomes critical when dealing with the production of vaccines or other potentially life-saving materials.
Safely meeting batch-to-batch repeatability
The danger to safety and reliability caused by a transmitter's inability to compensate for temperature while transmitting accurate pressure readings during the normal batch process puts a full batch quality at risk. When a device no longer functions as it should or stops completely, compliance with strict FDA rules is no longer met. Can a device still be trusted in an instance like this? A batch could be marked for non-conformance and thrown out, or the SIP cycle may need to be re-run to ensure the right production data is captured to move forward with a new batch.
But what if there were a pressure transmitter that could withstand repeated cleaning cycles without drifting or giving readings that are not correct? A hygienic pressure transmitter can do just that. These transmitters can recover quickly after a steam-in-place or washdown cycle and do not require constant recalibration or replacement. They are designed to handle the high heat of CIP cycles. Fewer recalibrations or inline replacements also mean a lower likelihood of batch delays (see Figure 2). Reliable repeatability batch-over-batch drives potential for significant cost savings and improvements in batch quality.
Pharma benefits from highly engineered solutions
The modern hygienic transmitter platform boasts some sophisticated capabilities, including easy-to-install sanitary fittings and specifications that meet 3-A Sanitary Standards, as well as the European Hygienic Engineering and Design Group (EHEDG) and American Society of Mechanical Engineers: Bioprocessing Equipment (ASME BPE) industry standards.
This new technology offering has been field-tested, and the hygienic transmitter platforms are specifically designed with the challenges of sanitary applications in mind. Robust batch-to-batch repeatability is possible due to built-in advanced sensors. This repeatable range is at ±0.02% of the upper range limit for 60 batches with less recovery time than legacy transmitters and longer maintenance intervals. These are valuable innovations that benefit the pharmaceutical industry specifically, though they have applications in other industries that use sterile and hygienic batch processes in their production facilities.
Having a device that can withstand repeated CIP and SIP cycles without compromising accuracy and reliability is not just a big benefit to meeting production schedules; it also represents a significant cost saving over the life of the device as it reduces the need for scheduled maintenance or replacement.
Brandon Haschke is a product manager focusing on Rosemount hygienic instrumentation at Emerson. His mission is to help operators get the most operational insight possible out of their pressure measurement points. Haschke holds degrees in chemistry and chemical engineering from the University of Minnesota.

Brandon Haschke | Product manager focusing on instrument manifolds for Emerson's Rosemount instrumentation business
Brandon Haschke is a product manager focusing on instrument manifolds for Emerson's Rosemount instrumentation business. He is currently focusing on helping operators in a variety of industries get the most out of their pressure measurement points. Brandon holds degrees in chemistry and chemical engineering from the University of Minnesota.